peptide helix drug development Peplix has developed new technology for making short stable peptide helix mimetics. The alpha helix is the most common regular structure in proteins and is a key part of many biological interactions. Critically these interactions are involved in diseases including cancer, diabetes, arthritis, osteoporosis and viral infections. A helix mimetic is a modified version of a helix and is potentially useful as a drug. Why make helix mimetics?
About the Technology The peplix technology has a number of attractive features: Below we describe some targets for helix mimetics followed by a brief discussion of methods for making mimetics. Examples of areas where helix mimetics can be useful are listed in the table below.
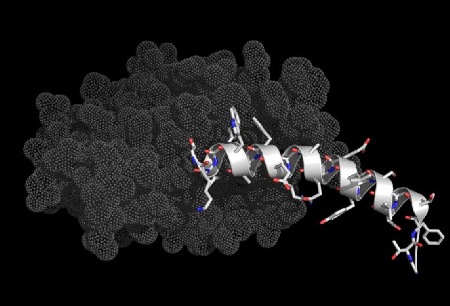
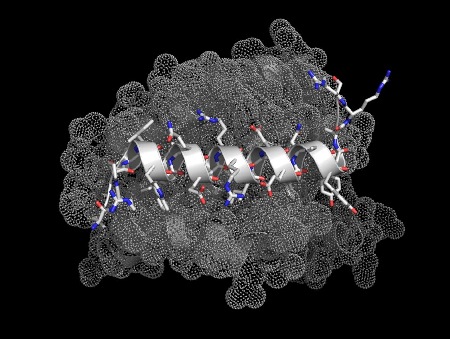
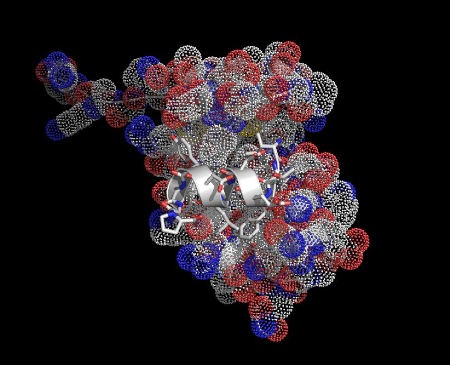
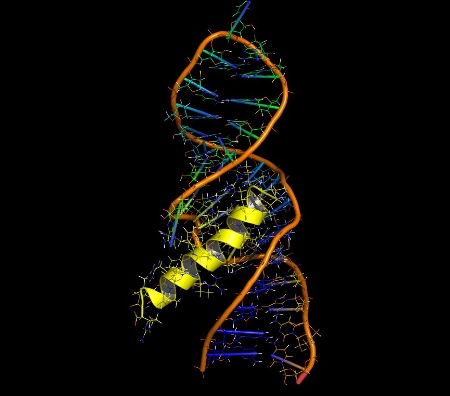
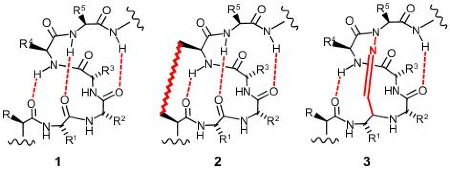
The illustrations below show helices in a range of significant biological interactions. All the structures are of real (rather than modeled) interactions derived from protein data bank structures as referenced. The main proteins are displayed with low detail in order to highlight the interacting helix.
The peptide hormone Glp-1 (white) bound to the extracellular part of its receptor (grey). Modified versions of this hormone are approved for the treatment of diabetes (liraglutide, byetta). The right hand end of the hormone binds to the transmembrane part of the receptor (not present in the crystal structure - transmembrane domains are difficult to crystallise). pdb: 3iol
The peptide Bim (white) bound to Bcl-2 (grey). This interaction is involved in the control of programmed cell death (apoptosis). Blocking the action of Bcl family proteins has been
shown to be useful in cancer therapy, this is an emerging field with
good potential. pdb: 3fdl
A 12 residue peptide inhibitor of HIV capsid assembly bound to a part of the HIV capsid protein.
A helical
peptide (Rev-ARM, yellow) bound to an RNA molecule (stem loop IIB), the highest
affinity binding site on Rev-responsive element (RRE). This interaction is a target for HIV(AIDS). pdb:1etg. About the Development of Helix Mimetics Approaches to making helix mimetics
The above images are 2D renderings of a short section of alpha helix. Structure 1 shows a normal helix with the three stabilising hydrogen bonds highlighted in red. The pattern of hydrogen bonds is what stabilises (and defines) an alpha helix. There are a number of approaches to making helix mimetics. One popular approach is to join two of the peptide sidechains together - this is illustrated in structure 2 above with the highlighted link between the R and R4 positions (these positions are closest together on consecutive loops of the helix). This approach has been successfully used by the company Aileron Therapeutics producing what they call stapled peptides. Structure
3 above illustrates another approach
to helix stabilisation where one of the hydrogen bonds (which are very weak) is
replaced by a chemical link. The link is
called a covalent hydrogen bond mimic
and the approach was first proposed in 1987.
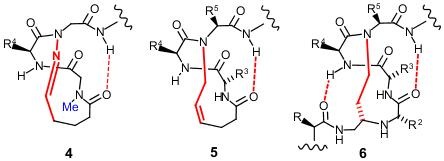
In practice making covalent hydrogen bond mimics (or hydrogen bond surrogates, "HBS") is difficult. Structure 4 shows an attempt at creating an HBS mimetic based on structure 3. The difficulty of
synthesis has meant that the lower part of the helix has been left out, all of
the sidechains except R4 have been left out, and a methyl group (highlighted in
blue) had to be added to aid cyclisation.
This hydrazone mimic (structures 3
and 4) has not seen much
practical application. The HBS 5 is similar to 4 as it can only be placed at the end of the helix and does not
include the R, R1 or R2 groups. Despite these limitations it has
seen some successful application.
There are other methods of making helix mimetics - notably non-peptide scaffolds such as terphenyl systems and related, and also peptoid and beta peptide approaches. These have been generally less successful than the more peptide based methods described above - the main reason is that the jump from the natural helix to non-peptides/peptoids is greater and tends to suffer from a correspondingly greater loss of activity which has proven difficult to recover by optimisation. |

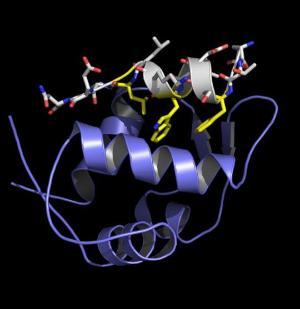 A fragment of the tumor suppressor protein p53 (white) bound to protein mdm2. Most cancers suppress the action of p53, reactivating it could be useful in cancer treatment.
A fragment of the tumor suppressor protein p53 (white) bound to protein mdm2. Most cancers suppress the action of p53, reactivating it could be useful in cancer treatment.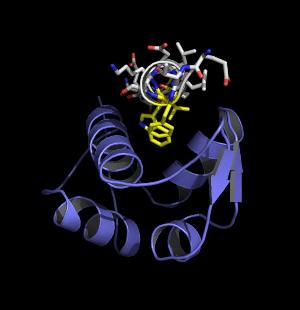 The same p53/mdm2 interaction viewed down the helical axis. The key interacting parts have been highlighted in yellow.
The same p53/mdm2 interaction viewed down the helical axis. The key interacting parts have been highlighted in yellow.